In the previous 10 months, there have been five similar warnings on pharmaceuticals made in India.
A cough syrup made in India was the subject of a substandard medicine notice from Iraq on Monday, the fifth such warning for Indian-made medications in the previous ten months, according to the World Health Organization (WHO).
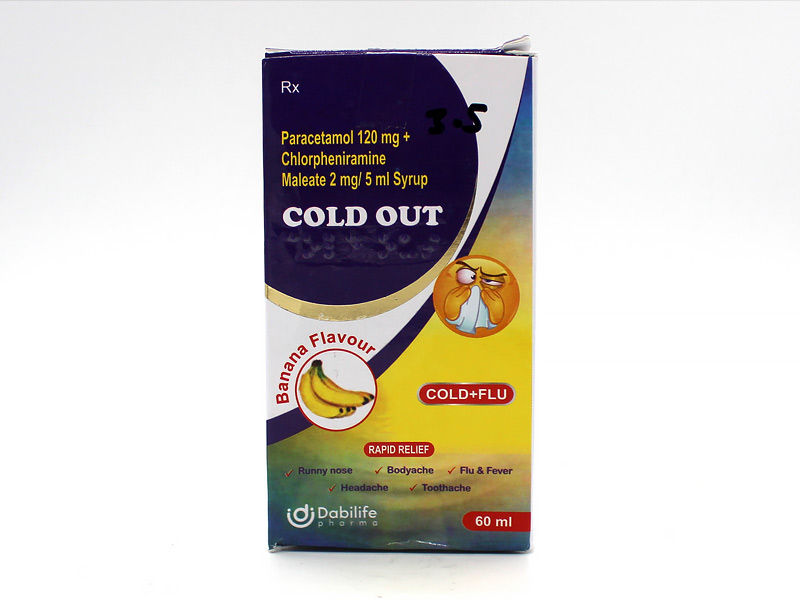
A sample of the ‘Cold Out’ Syrup was collected from one place in Iraq and given for laboratory testing. Diethylene glycol (0.25%) and ethylene glycol (2.1%) were discovered in the sample at levels that were deemed to be pollutants.
Diethylene glycol and ethylene glycol both have acceptable safety limits of no more than 0.10%.
Diethylene glycol & ethylene glycol are lethal poisons when consumed by humans.
The problematic batch of the Cold Out syrup was created by Fourrts (India) Laboratories Pvt Ltd in Tamil Nadu for Dabilife Pharma Pvt Ltd in Maharashtra.
According to a statement from the World Health Organization, this medical product alert concerns one sample of Cold Out syrup (Paracetamol & Chlorpheniramine Maleate), which was discovered in Iraq and reported to the organization on July 10, 2023, by a third party.
Syrups containing both paracetamol and chlorpheniramine are used to treat the symptoms of allergies and the common cold. In over a year, the WHO has examined at least five medicines made in India.

In The Gambia and Uzbekistan, respectively, the deaths of 66 and 18 children last year are thought to have been caused by cough syrups prepared in India. Recently, there have been more claims of contamination from cough medicine in Cameroon. Children in the US allegedly developed a severe eye infection after taking eye drops made in India.
According to the WHO, the product’s subpar batch was discovered in Iraq and when used, particularly on youngsters, might cause major harm or even death.
Toxic effects can include abdominal pain, nausea, vomiting, diarrhea, the inability to pass urine, migraines, altered mental status, and severe renal injury, which can be fatal.
Till the time of going to print, the Union health ministry had not responded to the development.
As a precaution, the UN health organization issued a thorough alert to governing bodies and the general public.
“WHO advises against using the affected product if you have it. You are encouraged to seek immediate medical counsel from a healthcare provider if you or someone you know has used the recalled product or may have done so and experienced a negative response or unanticipated side effects, the UN body stated in its caution.
Although this medical product alert only applies to one batch of the product, WHO urges additional testing and vigilance for the product as a whole out of an abundance of caution.
WHO suggested improved surveillance of informal and unregulated marketplaces as well as more diligence throughout the supply chains of nations and areas likely to be impacted by these items.
Also, Read Water Poisoning: 35-Year-Old Mom, Ashley Summers Dies
National regulatory and health agencies have been urged to alert WHO right once these subpar items are found in their respective nations.
The WHO recommended manufacturers of liquid dosage forms, particularly syrups that contain excipients such as propylene glycol, polyethylene glycol, sorbitol, and/or glycerin/glycerol, to conduct tests for pollutants like ethylene glycol and diethylene glycol before using them in medicines.
Healthcare practitioners have been encouraged to notify the National Regulatory Authorities or National Pharmacovigilance Centre of any suspicious occurrences of adverse events connected to the use of tainted drugs.

























